Biopharmaceutical characterization is crucial for ensuring drug safety, efficacy, and quality. It addresses challenges such as impurity detection, molecular structure analysis, and batch consistency. Issues like contamination and degradation can compromise drug performance and patient safety. Advanced techniques like MALDI-TOF MS provide precise molecular weight determination, impurity profiling, and metabolite analysis, offering robust solutions. These methods ensure regulatory compliance, enhance quality control and support the development of stable and effective pharmaceuticals, safeguarding public health and advancing medical innovation.
Protein/Peptide Characterization
Characterizing proteins and peptides is a crucial part of biopharmaceutical development, ensuring biologics' safety, efficacy, and quality. This involves in-depth analysis to determine proteins and peptides' molecular weight, structure, and composition. Advanced techniques such as MALDI-TOF MS achieve high precision and accuracy.
Key services in Protein/peptide characterization:
- Molecular weight determination
- Intact mass analysis
- Peptide mapping
- Post-translational modifications

MALDI Imaging Study
Imaging techniques like MALDI-IMS provide detailed spatial data about the location and distribution of proteins and peptides within biological tissues, offering insights into biological processes and disease mechanisms.
Tissue imaging in biopharmaceuticals:
Tissue imaging is an invaluable tool in the biopharmaceutical industry, providing critical insights into the distribution, localization, and activity of drug compounds within biological tissues. By integrating spatial and molecular information, tissue imaging enhances the understanding of drug mechanisms, efficacy, and safety, supporting the development of effective and targeted therapies.
Intact mass analysis by MALDI TOF
Intact mass analysis is a cornerstone technique in proteomics and biopharmaceutical characterization, enabling the precise determination of the molecular weight of proteins and other biomolecules in their native, undigested form. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI TOF) mass spectrometry stands out as a premier method for intact mass analysis, renowned for its sensitivity, accuracy, and high throughput capabilities. MALDI-TOF-MS provides accurate mass measurement and high-throughput analysis of intact biomolecules ranging from 1 to 100 kDa.
Applications Protein Characterization:
- Determines the molecular weight of therapeutic proteins, monoclonal antibodies, and other biologics to confirm identity and purity.
- Rapid Analysis: Facilitates quick data acquisition, suitable for high-throughput applications.
- Broad Applicability: Effective for various biomolecules, including proteins, peptides, lipids, and polysaccharides.
Small Molecule Analysis
Small molecule analysis is a crucial component of
biopharmaceutical characterization. It involves the
detailed examination of low molecular weight compounds
that often serve as active pharmaceutical ingredients
(APIs) or excipients. This analysis ensures that these
molecules meet the stringent quality, safety, and efficacy
standards for drug development and manufacturing. Mass
spectrometry provides precise and reliable molecular
weight and structural composition data, enabling
accurate identification and quantification of small
molecules.

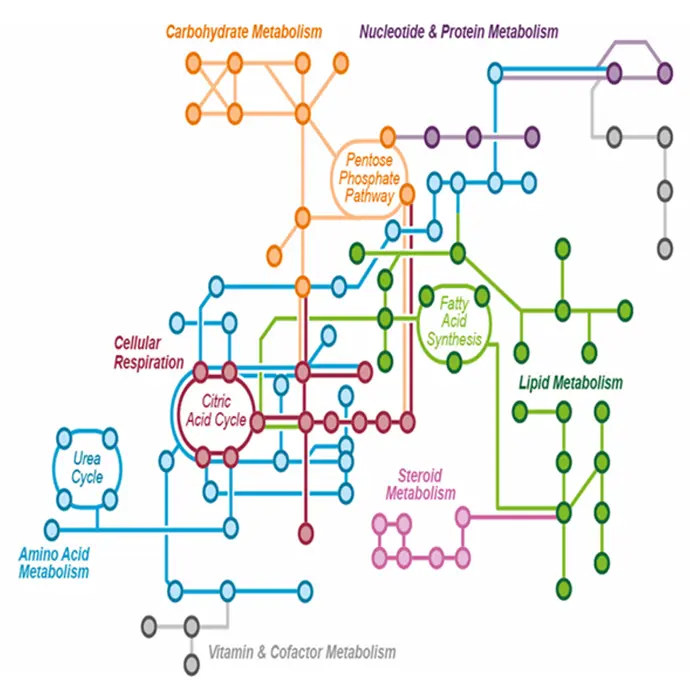
Metabolomic in Biopharma
Metabolomics has significant potential in pharmaceutical and clinical research, including the identification of new targets, the elucidation of the mechanism of action of new drugs, the characterization of safety and efficacy profiles, and the discovery of biomarkers for early disease diagnosis, prognosis, patient stratification, and treatment response monitoring.

Biomarker Discovery

Protein Characterization