DPYD Testing
Personalize fluoropyrimidine therapy with confidence by predicting risk, protecting patients, and preserving treatment efficacy.
Book Your Test TodaySeveral international guidelines (CPIC, AMP, EMA, FDA, ESMO) recommend DPYD testing before starting fluoropyrimidine treatment. It should no longer be optional; it should be standard of care.
Fluoropyrimidines such as 5-Fluorouracil (5-FU) and capecitabine are widely prescribed in oncology. However, globally, up to 30% of patients experience severe or life-threatening toxicity due to DPYD gene variants, which impair drug metabolism.

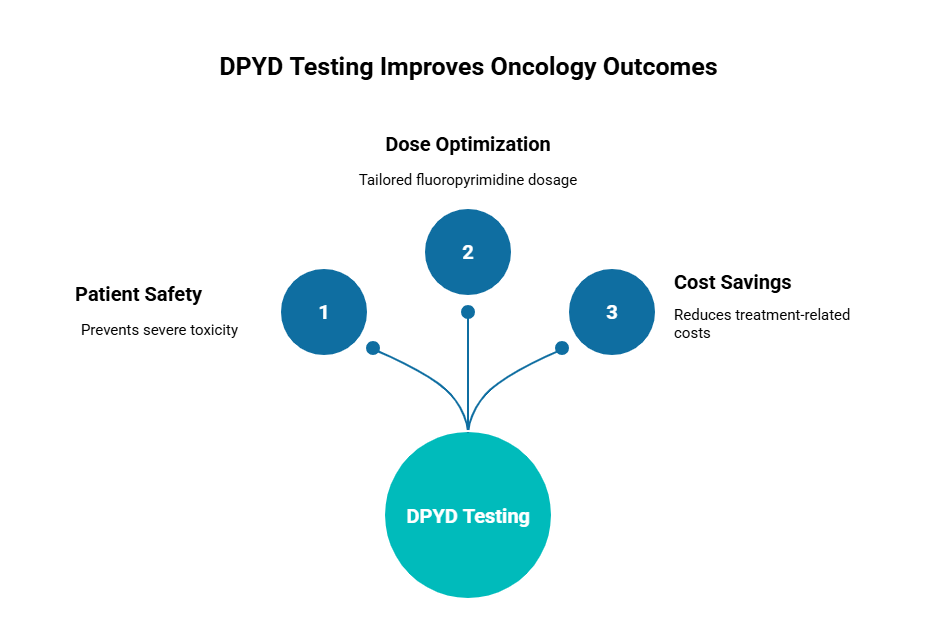
Why Adopt DPYD Testing in Oncology Practice?
DPYD testing should be integrated into every oncologist’s treatment algorithm for fluoropyrimidines. It is guideline-endorsed, clinically actionable, cost-saving, and most importantly, it prevents avoidable harm while maintaining therapeutic intent.
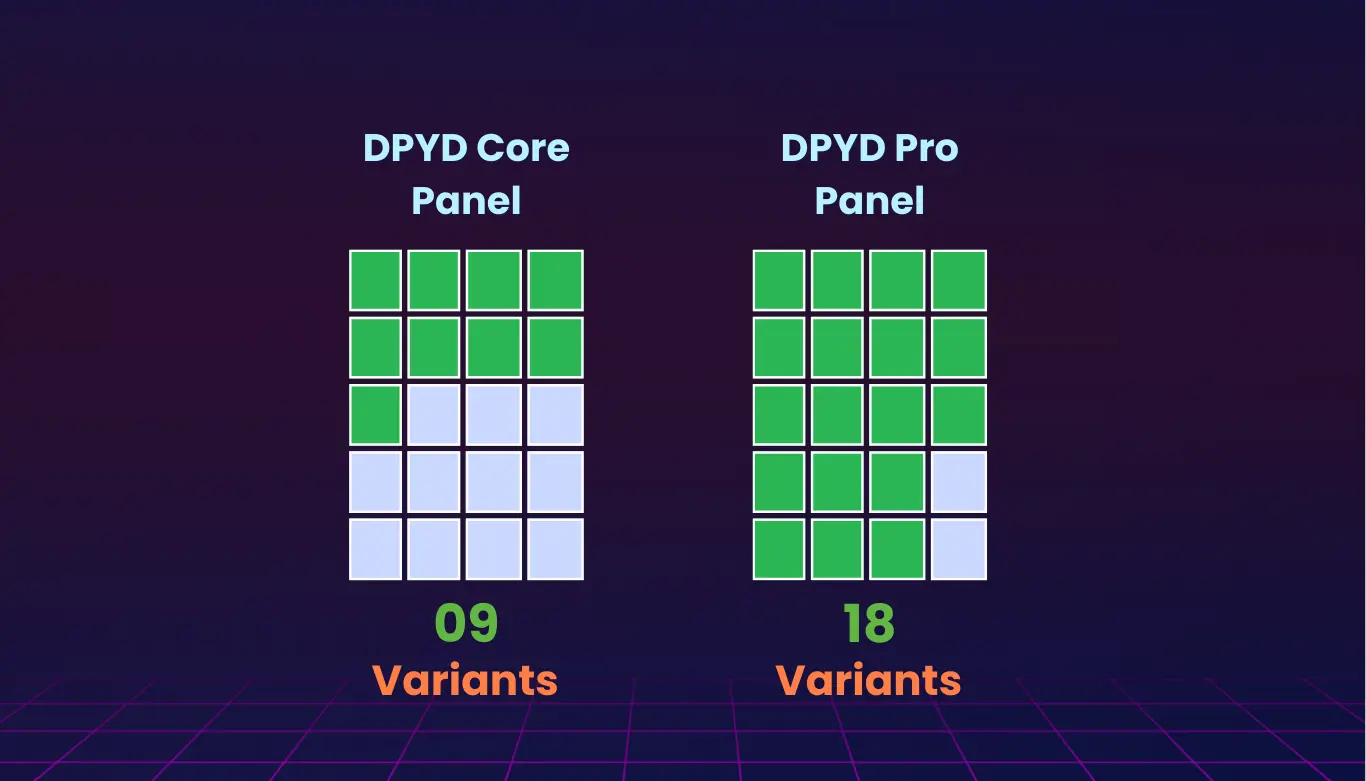
Panels Overview
Not all patients carry the same DPYD variants. Some harbor common guideline-recommended alleles, while others may carry rare or hybrid variants that standard panels can miss. To address this, we provide two levels of testing:
DPYD Core Panel
- Targets the most clinically validated and guideline-recommended 9 SNPs for variant analysis.
- Covers the alleles with strongest evidence for predicting severe fluoropyrimidine toxicity.
- Ideal for oncology centers adopting DPYD testing as part of routine chemotherapy workflows.
- Provides rapid, reliable risk stratification with direct impact on dose adjustment and treatment planning.
DPYD Pro Panel
- Extends beyond the essentials, offering broader variant coverage of 18 variants, including rare alleles and hybrid haplotypes.
- Designed for institutions treating genetically diverse patient populations.
- Improves clinical sensitivity, reducing the chance of missing less common but clinically relevant variants.
- Offers oncologists greater confidence when tailoring therapy in patients with complex or atypical genotypes.